PDS Story
Process Design Solutions (PDS), the solution provider for proEngQbD, is proud to be a Siemens Solution Partner for COMOS Industry Solutions. proEngQbD is powered by Siemens COMOS Software and is a fully integrated Engineering Information Management System that is specifically designed for the biopharmaceutical industry.
Process Design Solutions (PDS) provides client services and engineering solutions to the life science industry.

“Unlike many other engineering firms, Process Design Solutions (PDS) focuses our process engineering efforts on product quality and patient safety. Since 2009, Quality by Design (QbD) and its principles and concepts have driven our mission, vision, and values. PDS has invested in technology and innovation to drive QbD as a focal point of our core business. We have integrated this approach into our service model to deliver projects ‘Right First Time.’”
—Paul Stanovich, Managing Partner
COMOS -
Making Data Work
Better quality decision-making throughout the plant’s entire lifecycle

COMOS provides the process industry with a seamless flow of information based on a globally consistent database – integrated across all disciplines and all phases of a plant's lifecycle. This is possible thanks to the systematic application of object orientation. This means: In COMOS, all data relating to the same component form a unit – an object. Changes to object specifications are stored in the central COMOS database so that the updated data is available everywhere and at all times. Plant engineers and operators can access data that is always up-to-date – in real time and independent of time zones. That creates the basis for maximum decision-making reliability – and, thereby, for considerably greater productivity.
COMOS benefits at a glance:
- Integrated and consistent plant management
- Flexible application options in all disciplines and all lifecycle phases of a plant project
- Optimal integration and coordination of all plant subsections involved in planning and operation
- Reduction of process cycle times through close interaction between the engineering and operating phases
- Reduced data complexity through central storage of all plant information enables consistent access to all object-related data for any user
- Open system architecture for perfect adaptation to operation-specific requirements and integration of third-party systems into existing IT landscapes
The high flexibility of COMOS solutions can be individually tailored to your specific needs – and that's where we come in. As a company with extensive industry-specific know-how, we at apply all of our expertise to benefit your plant. We offer comprehensive consulting and technical support for customizations as well as training courses for COMOS users – for even more performance, quality and local presence.
More InformationCOMOS - Making Data Work
Better quality decision-making throughout the plant’s entire lifecycle
COMOS provides the process industry with a seamless flow of information based on a globally consistent database – integrated across all disciplines and all phases of a plant's lifecycle. This is possible thanks to the systematic application of object orientation. This means: In COMOS, all data relating to the same component form a unit – an object. Changes to object specifications are stored in the central COMOS database so that the updated data is available everywhere and at all times. Plant engineers and operators can access data that is always up-to-date – in real time and independent of time zones. That creates the basis for maximum decision-making reliability – and, thereby, for considerably greater productivity.
COMOS benefits at a glance:
- Integrated and consistent plant management
- Flexible application options in all disciplines and all lifecycle phases of a plant project
- Optimal integration and coordination of all plant subsections involved in planning and operation
- Reduction of process cycle times through close interaction between the engineering and operating phases
- Reduced data complexity through central storage of all plant information enables consistent access to all object-related data for any user
- Open system architecture for perfect adaptation to operation-specific requirements and integration of third-party systems into existing IT landscapes
The high flexibility of COMOS solutions can be individually tailored to your specific needs – and that's where we come in. As a company with extensive industry-specific know-how, we apply all of our expertise to benefit your plant. We offer comprehensive consulting and technical support for customizations as well as training courses for COMOS users – for even more performance, quality and local presence.
More InformationPDS Approach
Our unique approach applies the concepts of QbD to improve all aspects of the process engineering function.
PDS’s deep subject matter expertise combined with our innovative solution, proEngQbD, helps companies deliver projects Right First Time.
How We Do This
Listen. Plan. Deliver. By strategically focusing design efforts on product quality and patient safety, we extend the principles of QbD into the process engineering space for manufacturing systems, helping clients appropriately apply the principles of...
Quality Risk Management (QRM) via ICH Q9
Engineering Change Management (ECM) as a supporting process
Quality Systems (QS) to manage impact to critical aspects
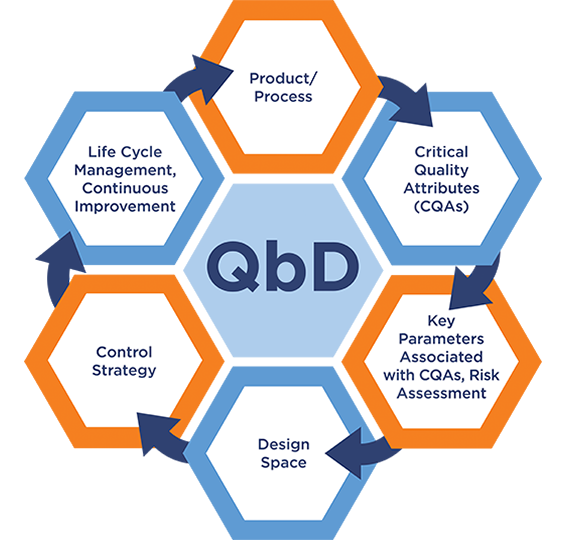
Quality by Design (QbD, ICH Q8) is the systematic approach to development that begins with predefined objectives and emphasizes product and process understanding and process control, based on sound science and quality risk management.


